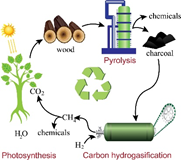
Carbon hydrogasification is the slowest reaction among all carbon-involved small molecule transformations. However, using mechanochemistry, a team at UNIST (South Korea) discovered that the reaction rate is dramatically enhanced by up to 4 orders of magnitude compared to the traditional thermal method.
Using internal field Cobalt NMR, we demonstrated that this extreme increase in reaction rate originates from the continuous activation of reactive carbon species with a cobalt catalyst though the formation of a cobalt carbide via mechanochemistry. This collaborative work between UNIST and the SIMM laboratory is expected to advance studies of carbon hydrogasification, and other solid-gas reactions crucial for the energy transition.
If you want to know more
G. Han, P. Zhang, P. Scholzen, H. Noh, M. Yang, D.H. Kweon, J. Jeon, Y.H. Kim, S. Kim, S. Han, A.S. Andreev, G. Lang, K. Ihm, F. Li, J. d’Espinose de Lacaillerie, J. Baek, Extreme Enhancement of Carbon Hydrogasification via Mechanochemistry, Angew Chem Int Ed. (2022).
https://doi.org/10.1002/anie.202117851.


