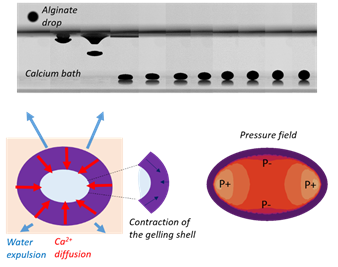
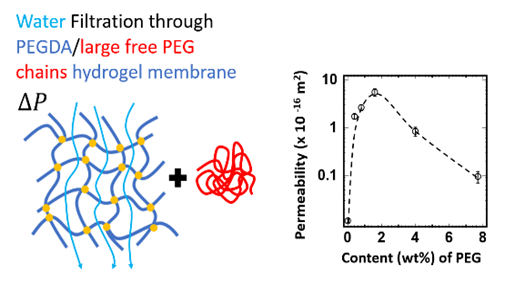
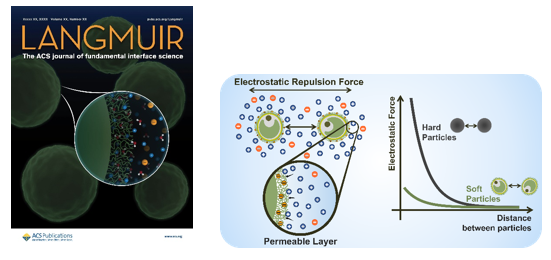
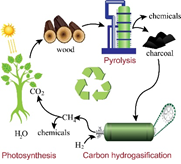
Edito
Sciences et Ingénierie de la Matière Molle - UMR7615
Les Sciences de la Matière Molle étudient des objets dont les propriétés macroscopiques sont fortement corrélées à leur structure mésoscopique. L’importance actuelle de cette discipline repose beaucoup sur la compréhension et l’élaboration maîtrisée d’objets aux échelles submicrométriques. Les concepts de base mûrissent depuis une quinzaine d’années, notamment sous l’impulsion de Pierre-Gilles de Gennes. Ils s’appuient sur des domaines aussi divers que la physique statistique, la mécanique, l’hydrodynamique, la physicochimie des surfaces ou la chimie. Ils irriguent maintenant des champs nouveaux comme la physique du vivant – marginale dans notre laboratoire – mais aussi un grand nombre de problématiques industrielles diverses, en particulier pour l’industrie chimique (matériaux polymères, ciments, colles, détergences…).
à la une
Utiliser l’AFM pour mesurer les propriétés mécaniques des nanoparticules lipidiques qui sont utilisées pour la vaccination contre la COVID
> Toutes les unes...
Actualités

Le prix ICMF 2025 du meilleur poster remis à Ange COMBROUZE pour ses travaux de thèse sur le temps de coalescence des bulles

Le prix Jeunes Talents France 2024 L’Oréal-UNESCO pour les femmes et la science remis à Paula ARAUJO-GOMES pour ses travaux de thèse sur la récolte des microalgues.
> Toutes les actualités...